Diacetyl Acyclovir
Diacetyl Acyclovir (Diacetylacyclovir)
Premium Quality Diacetyl Acyclovir (Diacetylacyclovir) – High-Purity Pharmaceutical Intermediate & Antiviral Drug Precursor for Acyclovir Manufacturing, Antiviral Drug Synthesis, API Production, Pharmaceutical Manufacturing & Research Applications
✅ Premium Pharmaceutical Intermediate – Superior Purity & Synthetic Properties
✅ Multiple Purity Grades – Various Quality Levels for Specific Pharmaceutical Applications
✅ Excellent Stability Properties – Superior Storage Stability, Controlled Composition, Precise Processing
✅ Multi-Application Compatible – Enhanced Pharmaceutical, Research, Manufacturing & Development Applications
✅ Quality Assured – Established Pharmaceutical Guidelines Supporting Optimal Performance
High-Quality Diacetyl Acyclovir (Diacetylacyclovir)
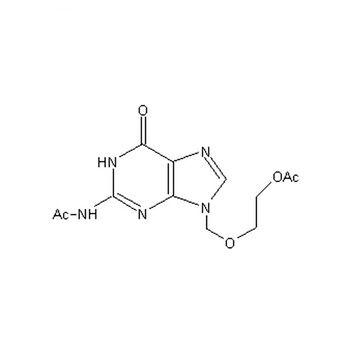
Diacetyl Acyclovir (Diacetylacyclovir), with CAS number 75128-73-3, is a white to almost white crystalline powder appearing as highly stable pharmaceutical intermediate with characteristic molecular structure and exceptional synthetic properties. Diacetyl Acyclovir represents one of the most important acyclovir intermediates, providing exceptional pharmaceutical utility, excellent chemical stability, superior synthetic potential, and outstanding research performance, making it essential for antiviral drug synthesis, API manufacturing, pharmaceutical research, drug development, and pharmaceutical applications requiring high-performance intermediate characteristics and consistent pharmaceutical behavior.
At Chemicals & Process Equipment Limited, we supply high-performance Diacetyl Acyclovir (Diacetylacyclovir) manufactured under stringent pharmaceutical quality control standards and international pharmaceutical, research, manufacturing, and development specifications. Our comprehensive product range includes various purity grades, quality levels, and specialized formulations meeting diverse requirements for pharmaceutical synthesis, antiviral drug manufacturing, API production, research applications, and specialized pharmaceutical processing across global pharmaceutical, research, biotechnology, and drug development markets.
✅ Advanced Pharmaceutical Technology – Optimized Purity Levels & Multi-Application Compatibility
✅ Consistent Quality Standards – Multiple Grade Classifications with Comprehensive Pharmaceutical Documentation
✅ Essential Pharmaceutical Intermediate – Superior Antiviral, Research & Manufacturing Performance
✅ Multi-Industry Applications – Pharmaceutical, Research, Biotechnology, API & Drug Development Systems
✅ Technical Support Package – Complete Processing Guidance & Application Optimization
The global acyclovir market was valued at approximately USD 1.2 billion in 2023 and is projected to reach USD 1.8 billion by 2030, growing at a CAGR of 5.8% during the forecast period. Market expansion is driven by increasing antiviral drug demand, growing herpes treatment requirements, expanding pharmaceutical manufacturing, rising research activities, and advanced drug development requiring high-performance pharmaceutical intermediates.
Technical Specifications & Diacetyl Acyclovir Properties
| Property | Pharmaceutical Grade | Research Grade | API Grade | Technical Grade | USP Grade | Manufacturing Grade |
|---|---|---|---|---|---|---|
| Purity (%) | 98.5-99.5 | 99.0-99.8 | 99.5-99.9 | 96-98 | 99.0-99.5 | 97-99 |
| Diacetyl Acyclovir (%) | 98.5-99.5 | 99.0-99.8 | 99.5-99.9 | 96-98 | 99.0-99.5 | 97-99 |
| Related Substances (%) | <1.5 | <1.0 | <0.5 | <4.0 | <1.0 | <3.0 |
| Acyclovir (%) | <0.5 | <0.3 | <0.2 | <1.0 | <0.3 | <0.8 |
| Acetyl Impurities (%) | <0.5 | <0.3 | <0.2 | <1.0 | <0.3 | <0.8 |
| Water Content (%) | <0.5 | <0.3 | <0.2 | <1.0 | <0.3 | <0.8 |
| Heavy Metals (ppm) | <20 | <10 | <5 | <50 | <10 | <30 |
| Lead (Pb) (ppm) | <5 | <2 | <1 | <10 | <2 | <8 |
| Mercury (Hg) (ppm) | <1 | <0.5 | <0.2 | <2 | <0.5 | <1.5 |
| Arsenic (As) (ppm) | <3 | <1 | <0.5 | <5 | <1 | <4 |
| Cadmium (Cd) (ppm) | <1 | <0.5 | <0.2 | <2 | <0.5 | <1.5 |
| Residual Solvents (ppm) | <500 | <300 | <200 | <1000 | <300 | <800 |
| Methanol (ppm) | <300 | <200 | <100 | <500 | <200 | <400 |
| Ethanol (ppm) | <500 | <300 | <200 | <1000 | <300 | <800 |
| Acetone (ppm) | <500 | <300 | <200 | <1000 | <300 | <800 |
| Dichloromethane (ppm) | <60 | <30 | <20 | <100 | <30 | <80 |
| Molecular Weight | 309.28 | 309.28 | 309.28 | 309.28 | 309.28 | 309.28 |
| Molecular Formula | C₁₂H₁₅N₅O₅ | C₁₂H₁₅N₅O₅ | C₁₂H₁₅N₅O₅ | C₁₂H₁₅N₅O₅ | C₁₂H₁₅N₅O₅ | C₁₂H₁₅N₅O₅ |
| Melting Point (°C) | 202-206 | 204-206 | 204-205 | 200-208 | 203-206 | 201-207 |
| Density (g/cm³ at 20°C) | 1.52-1.54 | 1.53-1.54 | 1.530-1.535 | 1.50-1.55 | 1.52-1.54 | 1.51-1.54 |
| Refractive Index (nD20) | 1.652-1.656 | 1.653-1.655 | 1.654 | 1.650-1.658 | 1.653-1.655 | 1.651-1.657 |
| Solubility in Water (mg/mL) | 0.2-0.5 | 0.3-0.4 | 0.35 | 0.1-0.6 | 0.3-0.4 | 0.2-0.5 |
| Solubility in DMSO (mg/mL) | >50 | >50 | >50 | >50 | >50 | >50 |
| Solubility in Methanol (mg/mL) | 10-20 | 15-18 | 16 | 8-25 | 15-18 | 12-20 |
| pH (1% DMSO solution) | 6.0-8.0 | 6.5-7.5 | 7.0 | 5.5-8.5 | 6.5-7.5 | 6.0-8.0 |
| Loss on Drying (%) | <0.5 | <0.3 | <0.2 | <1.0 | <0.3 | <0.8 |
| Residue on Ignition (%) | <0.1 | <0.05 | <0.02 | <0.2 | <0.05 | <0.15 |
| Particle Size (μm) | 50-200 | 75-150 | 80-120 | 25-300 | 75-150 | 40-250 |
| Bulk Density (g/mL) | 0.4-0.6 | 0.45-0.55 | 0.50 | 0.3-0.7 | 0.45-0.55 | 0.35-0.65 |
| Tapped Density (g/mL) | 0.6-0.8 | 0.65-0.75 | 0.70 | 0.5-0.9 | 0.65-0.75 | 0.55-0.85 |
| Surface Area (m²/g) | 2-8 | 3-6 | 4-5 | 1-12 | 3-6 | 2-10 |
| Thermal Stability (°C) | >200 | >200 | >200 | >190 | >200 | >195 |
| Decomposition Temperature (°C) | >250 | >250 | >250 | >240 | >250 | >245 |
| UV Absorption (280 nm) | Characteristic | Characteristic | Characteristic | Characteristic | Characteristic | Characteristic |
| FTIR Identification | Conforms | Conforms | Conforms | Conforms | Conforms | Conforms |
| NMR Identification (¹H) | Conforms | Conforms | Conforms | Conforms | Conforms | Conforms |
| MS Identification | Conforms | Conforms | Conforms | Conforms | Conforms | Conforms |
| Optical Rotation | Nil | Nil | Nil | Nil | Nil | Nil |
| Color (Gardner) | <2 | <1 | <1 | <4 | <1 | <3 |
| Appearance | White powder | White powder | White powder | Off-white powder | White powder | White powder |
| Odor | Odorless | Odorless | Odorless | Slight | Odorless | Slight |
| Hygroscopicity | Low | Low | Low | Moderate | Low | Low |
| Photostability | Good | Good | Excellent | Fair | Good | Good |
| Oxidation Stability | Good | Good | Excellent | Fair | Good | Good |
| Storage Stability | Excellent | Excellent | Excellent | Good | Excellent | Good |
| Container Compatibility | Glass/HDPE | Glass/HDPE | Borosilicate glass | HDPE/PP | Glass/HDPE | HDPE/PP |
| Electrostatic Properties | Low | Low | Low | Moderate | Low | Low |
| Compressibility Index | 25-35 | 28-32 | 30 | 20-40 | 28-32 | 25-40 |
| Hausner Ratio | 1.3-1.8 | 1.4-1.6 | 1.5 | 1.2-2.0 | 1.4-1.6 | 1.3-1.9 |
| Flow Properties | Fair to Good | Good | Good | Poor to Fair | Good | Fair to Good |
| Crystalline Form | Form I | Form I | Form I | Polymorphic | Form I | Form I |
| Crystal Structure | Monoclinic | Monoclinic | Monoclinic | Variable | Monoclinic | Monoclinic |
| Polymorphism | Stable | Stable | Stable | Variable | Stable | Stable |
| Partition Coefficient (log P) | 1.2-1.8 | 1.4-1.6 | 1.5 | 1.0-2.0 | 1.4-1.6 | 1.2-1.8 |
| Bioavailability (%) | N/A | N/A | N/A | N/A | N/A | N/A |
| Protein Binding (%) | N/A | N/A | N/A | N/A | N/A | N/A |
| Metabolic Stability | Hydrolyzed | Hydrolyzed | Hydrolyzed | Hydrolyzed | Hydrolyzed | Hydrolyzed |
| CAS Number | 75128-73-3 | 75128-73-3 | 75128-73-3 | 75128-73-3 | 75128-73-3 | 75128-73-3 |
| EINECS Number | 278-295-8 | 278-295-8 | 278-295-8 | 278-295-8 | 278-295-8 | 278-295-8 |
| MDL Number | MFCD00869377 | MFCD00869377 | MFCD00869377 | MFCD00869377 | MFCD00869377 | MFCD00869377 |
| PubChem CID | 135398513 | 135398513 | 135398513 | 135398513 | 135398513 | 135398513 |
| ChemSpider ID | 21106257 | 21106257 | 21106257 | 21106257 | 21106257 | 21106257 |
| RTECS Number | GU4725000 | GU4725000 | GU4725000 | GU4725000 | GU4725000 | GU4725000 |
| UN Number | Not regulated | Not regulated | Not regulated | Not regulated | Not regulated | Not regulated |
| Storage Temperature (°C) | 2-8 | 2-8 | 2-8 | 15-25 | 2-8 | 15-25 |
| Relative Humidity (%) | <60 | <50 | <40 | <70 | <50 | <65 |
| Shelf Life (months) | 36 | 48 | 60 | 24 | 48 | 36 |
| Container Material | Amber glass | Amber glass | Borosilicate glass | HDPE | Amber glass | HDPE |
| Packaging Sizes | 1g-1kg | 100mg-500g | 1g-100g | 25g-25kg | 1g-1kg | 100g-10kg |
| Inert Atmosphere | Recommended | Recommended | Required | Optional | Recommended | Optional |
| Quality Certification | Pharmaceutical | Research | API | Technical | USP | Manufacturing |
Available Grade Types:
- Pharmaceutical Grade for drug manufacturing and API synthesis
- Research Grade for academic and scientific research applications
- API Grade for active pharmaceutical ingredient production
- Technical Grade for general industrial applications
- USP Grade for United States Pharmacopeia standards
- Manufacturing Grade for large-scale pharmaceutical production
- Custom Grade for specific application requirements
- Reference Standard for analytical and quality control
- Impurity Standard for method development
- High Purity Grade for critical applications
- Analytical Grade for laboratory testing
- GMP Grade for good manufacturing practices
- Reagent Grade for chemical synthesis
- Electronic Grade for specialized applications
Specialized Processing Solutions:
- Purity optimization for specific pharmaceutical applications
- Impurity profiling and characterization
- Custom synthesis and manufacturing
- Analytical method development
- Stability studies and shelf-life determination
- Packaging optimization consultation
- Quality testing and validation programs
- Regulatory support and documentation
- Technical consultation services
- Scale-up and commercial manufacturing
Pharmaceutical Applications
Acyclovir Manufacturing & API Synthesis Pharmaceutical manufacturers utilize Diacetyl Acyclovir as a key intermediate for acyclovir production, antiviral drug synthesis, and API manufacturing requiring pharmaceutical specifications. Drug companies employ Diacetyl Acyclovir for acyclovir synthesis, serving as an essential intermediate in the production process, requiring high purity, consistent quality, and regulatory compliance for antiviral drug manufacturing processes.
Antiviral Drug Development & Research Pharmaceutical research companies use Diacetyl Acyclovir for antiviral drug development, herpes treatment research, and therapeutic compound evaluation requiring research specifications. Biotechnology companies employ Diacetyl Acyclovir for drug discovery, mechanism studies, and pharmaceutical research requiring analytical purity, research grade quality, and scientific protocols.
Generic Drug Manufacturing Generic pharmaceutical manufacturers utilize Diacetyl Acyclovir for generic acyclovir production, cost-effective drug manufacturing, and pharmaceutical equivalence requiring generic specifications. Generic drug companies employ Diacetyl Acyclovir for bioequivalent formulations, regulatory submissions, and generic applications requiring pharmaceutical standards and regulatory compliance.
Formulation Development & Optimization Pharmaceutical formulators use Diacetyl Acyclovir for dosage form development, drug delivery research, and formulation optimization requiring formulation specifications. Research institutions employ Diacetyl Acyclovir for tablet formulations, oral suspensions, and pharmaceutical applications requiring formulation expertise, stability studies, and quality assurance.
Research & Development Applications
Antiviral Research & Virology Studies Research institutions utilize Diacetyl Acyclovir for antiviral mechanism research, viral replication studies, and drug resistance evaluation requiring research specifications. Academic laboratories employ Diacetyl Acyclovir for herpes simplex virus research, studying the mechanisms of antiviral drug action and resistance development, requiring research grade purity, analytical standards, and safety protocols.
Medicinal Chemistry & Drug Design Medicinal chemists use Diacetyl Acyclovir for structure-activity relationship studies, drug design research, and chemical modification requiring medicinal chemistry specifications. Pharmaceutical research companies employ Diacetyl Acyclovir for lead optimization, analog synthesis, and drug development requiring synthetic chemistry expertise and analytical characterization.
Pharmacokinetic & Metabolism Studies Pharmacology laboratories utilize Diacetyl Acyclovir for ADME studies, metabolic pathway research, and pharmacokinetic evaluation requiring pharmacology specifications. Contract research organizations employ Diacetyl Acyclovir for preclinical studies, drug metabolism, and pharmacological applications requiring analytical methods and regulatory compliance.
Analytical Method Development Analytical laboratories use Diacetyl Acyclovir for method validation, analytical testing, and quality control procedures requiring analytical specifications. Testing laboratories employ Diacetyl Acyclovir for HPLC methods, mass spectrometry, and analytical applications requiring reference standards, method development, and validation protocols.
Manufacturing & Production Applications
Large-Scale Pharmaceutical Manufacturing Commercial pharmaceutical manufacturers utilize Diacetyl Acyclovir for bulk drug production, industrial synthesis, and commercial manufacturing requiring manufacturing specifications. Pharmaceutical companies employ Diacetyl Acyclovir for process optimization, cost reduction, and manufacturing applications requiring scale-up expertise, quality control, and regulatory compliance.
Contract Manufacturing Services Contract manufacturers use Diacetyl Acyclovir for custom synthesis, toll manufacturing, and pharmaceutical production requiring contract manufacturing specifications. CDMO companies employ Diacetyl Acyclovir for client projects, regulatory manufacturing, and commercial applications requiring GMP compliance, quality systems, and regulatory documentation.
API Production & Purification API manufacturers utilize Diacetyl Acyclovir for intermediate processing, purification steps, and API production requiring API specifications. Pharmaceutical ingredient companies employ Diacetyl Acyclovir for multi-step synthesis, process development, and API applications requiring chemical expertise, purification technology, and quality assurance.
Process Development & Optimization Process development teams use Diacetyl Acyclovir for route optimization, cost reduction, and process improvement requiring process development specifications. Chemical engineers employ Diacetyl Acyclovir for reaction optimization, yield improvement, and process applications requiring engineering expertise, analytical support, and scale-up capabilities.
Quality Control & Analytical Applications
Reference Standards & Calibration Quality control laboratories utilize Diacetyl Acyclovir as reference standards, analytical calibrants, and testing materials requiring reference standard specifications. Pharmaceutical companies employ Diacetyl Acyclovir for method validation, system suitability, and analytical applications requiring certified reference materials, traceability, and analytical accuracy.
Impurity Profiling & Characterization Analytical laboratories use Diacetyl Acyclovir for impurity identification, degradation studies, and analytical characterization requiring analytical specifications. Pharmaceutical companies employ Diacetyl Acyclovir for stability testing, forced degradation, and quality applications requiring analytical expertise, method development, and regulatory compliance.
Stability Studies & Shelf-Life Determination Stability laboratories utilize Diacetyl Acyclovir for accelerated studies, long-term stability, and shelf-life evaluation requiring stability specifications. Pharmaceutical companies employ Diacetyl Acyclovir for ICH studies, registration batches, and stability applications requiring controlled conditions, analytical testing, and regulatory documentation.
Method Validation & System Suitability Analytical method developers use Diacetyl Acyclovir for validation studies, system suitability, and analytical method development requiring validation specifications. Contract testing laboratories employ Diacetyl Acyclovir for method transfer, cross-validation, and analytical applications requiring validation protocols, documentation, and regulatory compliance.
Regulatory & Compliance Applications
Drug Registration & Regulatory Submissions Regulatory affairs teams utilize Diacetyl Acyclovir for drug master files, regulatory dossiers, and registration submissions requiring regulatory specifications. Pharmaceutical companies employ Diacetyl Acyclovir for FDA submissions, EMA filings, and regulatory applications requiring regulatory documentation, quality data, and compliance protocols.
Good Manufacturing Practice Compliance GMP compliance teams use Diacetyl Acyclovir for validated processes, quality systems, and GMP manufacturing requiring GMP specifications. Pharmaceutical manufacturers employ Diacetyl Acyclovir for audit preparation, inspection readiness, and GMP applications requiring quality systems, documentation, and regulatory compliance.
Quality Assurance & Documentation Quality assurance departments utilize Diacetyl Acyclovir for quality control, batch release, and documentation requirements requiring QA specifications. Pharmaceutical companies employ Diacetyl Acyclovir for quality reviews, deviation investigations, and QA applications requiring quality systems, change control, and regulatory documentation.
International Harmonization Standards International regulatory teams use Diacetyl Acyclovir for ICH compliance, global harmonization, and international standards requiring harmonization specifications. Global pharmaceutical companies employ Diacetyl Acyclovir for multiple market submissions, regulatory harmonization, and international applications requiring global regulatory expertise and compliance protocols.
Quality Control & Testing Procedures
Comprehensive Pharmaceutical Quality Assurance Every production batch undergoes extensive testing to ensure compliance with pharmaceutical standards, USP requirements, ICH guidelines, and international pharmaceutical specifications. Our quality control laboratory employs advanced analytical techniques including HPLC analysis, mass spectrometry, NMR spectroscopy, and comprehensive purity testing.
Quality assurance procedures include:
- Purity determination using validated HPLC methods
- Related substances analysis using gradient HPLC
- Water content analysis using Karl Fischer titration
- Heavy metals testing using ICP-MS methods
- Residual solvents analysis using GC-MS methods
- Melting point determination using capillary methods
- Loss on drying using thermogravimetric analysis
- Residue on ignition using muffle furnace methods
- Particle size analysis using laser diffraction
- Bulk and tapped density measurement
- FTIR spectroscopic identification
- ¹H NMR spectroscopic confirmation
- UV-Vis spectrophotometric analysis
- Mass spectrometric identification
- Crystalline form analysis using X-ray diffraction
- Thermal analysis using DSC methods
- Moisture content determination
- pH measurement in suitable solvents
- Optical rotation measurement (where applicable)
- Color assessment using Gardner standards
- Microbial contamination testing (total aerobic count)
- Endotoxin testing (where required)
- Genotoxicity assessment for impurities
- Stability studies under ICH conditions
- Accelerated stability testing
- Photostability testing according to ICH Q1B
- Forced degradation studies
- Container compatibility testing
- Extractables and leachables testing
- Method validation according to ICH Q2
- System suitability testing
- Reference standard qualification
- Analytical method transfer
- Cross-validation studies
- Uncertainty of measurement evaluation
- Statistical analysis of analytical data
- Trend analysis and control charting
- Out of specification investigations
- Change control documentation
- Batch release procedures
- Certificate of analysis preparation
- Regulatory compliance verification
- GMP documentation and records
- Quality management system compliance
- Risk assessment and management
- Continuous improvement initiatives
Storage & Handling Procedures Implement proper pharmaceutical storage conditions including refrigerated storage at 2-8°C, protection from light using amber containers, protection from moisture below 50% RH, and proper handling procedures using appropriate pharmaceutical protocols for maintaining Diacetyl Acyclovir quality and pharmaceutical integrity throughout storage and handling periods.
For comprehensive information about diacetyl acyclovir properties, pharmaceutical applications, analytical methods, and research data, you can reference this detailed PubChem Database resource for Acyclovir and related compounds which provides extensive coverage of chemical properties, biological activities, safety data, and research information from leading pharmaceutical institutions and scientific literature.
Market Applications & Industry Trends
Antiviral Drug Market Growth Growing antiviral medication demand, increasing herpes treatment requirements, expanding generic drug markets, and rising pharmaceutical manufacturing drive continued demand for high-purity diacetyl acyclovir in pharmaceutical applications requiring reliable synthetic intermediates, regulatory compliance, and quality assurance.
Pharmaceutical Manufacturing Expansion Developing pharmaceutical markets, increasing API manufacturing, expanding generic drug production, and growing contract manufacturing drive adoption of pharmaceutical-grade diacetyl acyclovir for manufacturing applications requiring cost-effectiveness, reliable supply, and pharmaceutical scale processing.
Research & Development Investment Growing pharmaceutical research, increasing R&D investments, expanding drug discovery activities, and rising academic research drive growth in research-grade diacetyl acyclovir applications requiring analytical purity, research reliability, and safety protocols for scientific advancement.
Regulatory & Quality Requirements Expanding pharmaceutical regulations, increasing quality standards, and growing compliance requirements support demand for high-purity diacetyl acyclovir and comprehensive quality documentation in various applications requiring regulatory responsibility and pharmaceutical compliance.
Environmental & Regulatory Compliance
Pharmaceutical Manufacturing Standards Diacetyl Acyclovir complies with pharmaceutical manufacturing standards, USP monographs, and international pharmaceutical guidelines ensuring appropriate use in pharmaceutical applications with established purity criteria and quality protocols for drug manufacturing.
Good Manufacturing Practice Compliance Adherence to GMP guidelines, pharmaceutical quality standards, and international manufacturing protocols ensures reliable use in pharmaceutical applications with established manufacturing criteria and quality systems for pharmaceutical production.
Research Safety Standards Compliance with laboratory safety regulations, research guidelines, and institutional safety standards ensures reliable use in research applications with established safety margins and protective measures for research personnel.
Environmental & Waste Management Environmental compliance includes pharmaceutical waste management protocols, solvent recovery procedures, and environmental protection initiatives ensuring responsible handling with consideration for environmental impact and regulatory compliance for pharmaceutical applications.
Regulatory Documentation & Support Comprehensive regulatory documentation including drug master files, analytical methods, stability data, and quality protocols ensures regulatory compliance with established pharmaceutical requirements and regulatory submission support.
Performance Advantages & Technical Benefits
✔ Essential Pharmaceutical Intermediate – Critical precursor for acyclovir manufacturing and antiviral drug production
✔ High Pharmaceutical Purity – Superior purity grades for pharmaceutical and API applications
✔ Excellent Stability – Superior storage stability and chemical compatibility
✔ Versatile Applications – Multi-application compatibility across pharmaceutical sectors
✔ Technical Excellence – Proven performance in pharmaceutical synthesis and manufacturing
✔ Regulatory Compliance – Comprehensive pharmaceutical documentation and quality data
✔ Quality Assurance – Advanced analytical testing and pharmaceutical quality control
✔ Expert Support – Complete technical guidance and pharmaceutical consultation
✔ Reliable Supply – Consistent availability and dependable pharmaceutical delivery
✔ Safety Focus – Comprehensive pharmaceutical safety protocols and handling guidelines
✔ Custom Solutions – Tailored grades and specifications for unique pharmaceutical applications
✔ Global Standards – International pharmaceutical quality standards and certification
✔ Cost Effectiveness – Competitive pricing for premium pharmaceutical quality
✔ Research Support – Academic and industrial research collaboration opportunities
✔ Innovation Partnership – Research and development collaboration for pharmaceutical advancement
✔ Technical Documentation – Complete certificates of analysis and pharmaceutical data
Our Diacetyl Acyclovir (Diacetylacyclovir) products represent industry-leading standards in pharmaceutical intermediate technology and pharmaceutical chemical solutions, specifically manufactured for demanding pharmaceutical applications, research requirements, API production, and specialized pharmaceutical applications requiring exceptional purity characteristics, reliable synthetic behavior, and comprehensive pharmaceutical compliance. We provide complete technical support, pharmaceutical guidance, and application consultation to ensure optimal results across your specialized pharmaceutical, research, manufacturing, or development applications.
For detailed specifications, technical data sheets, or application-specific recommendations, contact our specialized pharmaceutical intermediate division. We offer complete support including grade selection, analytical methods, stability data, and technical assistance to maximize effectiveness in your pharmaceutical, research, API, or development applications.
For more information about our products, feel free to Contact Us today.



















Reviews
There are no reviews yet.